News feed
BME research may offer an alternative way to produce hydrogen
2023. 04. 28.Áron Kozák, student of mechatronical engineering, recipient of the Gábor Dénes Scientific Student Scholarship, is investigating energy management of hydrogen production in his project.
 |
“It was my first time in a scientific student competition, and I was delighted when the jury deemed my paper to be one of the best among several high quality entries. I may represent BME in the spring OTDK, which is also a great honour, while the scholarship is a special professional recognition, which makes me immensely proud,” said Áron Kozák, BSc student of mechatronical engineering at the BME Faculty of Mechanical Engineering, one of the recent winners of the Gábor Dénes Scholarship. The title of his thesis: Bubble dynamics in hydrogen production: numerical optimization of energy efficiency (supervisors: associate professor Ferenc Hegedűs and PhD student Csanád Kalmár, both from the Department of Hydrodinamic Systems, BME GPK).
|
|
NOVOFER Foundation and BME established the Gábor Dénes Scholarship in July 2001, in order to deepen the professional knowledge of the university students and to increase their commitment to science. The scholarship, which is awarded with a certificate of honour and a one-off prize of HUF 100,000, is presented to the selected students once a year at the Gábor Dénes Award ceremony, at the end of the year.
Tibor Czigány (Rector, BME), Andrea Toldy - Gábor Dénes Award 2022, András Sóki and Áron Kozák (Gábor Dénes Scholarship 2022) Recipients of the Gábor Dénes Scholarship in 2022: Kozák Áron BSc student of mechatronical engineering (BME GPK), the title of his thesis: Bubble dynamics in hydrogen production: numerical optimization of energy efficiency András Sóki, BSc student of electrical engineering (BME VIK), the title of his thesis: Integration of quantum channels into existing optical networks. |
Áron Kozák chose the BSc in Mechatronics Engineering programme at BME GPK. He was inspired by the fact that as a student of this programme he will have the opportunity to explore several disciplines where programming skills play an important part, which he will use during his studies and hopefully as a graduate engineer.
He was introduced to his research topic through an elective course on the foundation of high-performance computing (Introduction to dynamical systems and their hardware-optimised numerical methods), announced by his future advisor, Ferenc Hegedűs, with the aim of involving students in the research of the same subject. “We had to solve the programming challenges of a freely chosen problem,” Áron Kozák recalled the requirements of the course. “I was immensely interested in it, so I created a computer program that plots the Mandelbrot set. All new students of mechatronical engineering students have to do this as part of the compulsory course, but I was able to do it orders of magnitude faster, using what I had learned in class. This prompted the request from Ferenc Hegedűs, who was looking for students with programming skills for his academic work.”
Áron Kozák’s Mandelbrot set plotter
Áron Kozák joined the department’s sonochemical research a couple of months later, in which the researchers were trying to use tiny bubbles of just a few micrometers in diameter, as chemical reactors. Predetermined micron-sized bubbles containing oxygen or argon in a liquid (e.g. water) are created during hydrogen production. Technologies already exist for this process, which were previously developed for the production of metal foams or pharmaceuticals. Usually sinusoidal ultrasound is used to vibrate the bubbles, which expand and then suddenly collapse as they radially pulse. “During expansion water can evaporate into the bubble, and the bubble then undergoes a significant increase in pressure and temperature of up to several thousand degrees Kelvin. The water dissociates into hydrogen and oxygen under these conditions, in other words hydrogen is produced,” Áron Kozák described the details of the tests. The Gábor Dénes Scholarship student simulates the chemical processes in the bubbles on a computer and proposes solutions to optimize the energy efficiency of the method. Áron Kozák explained that this research also contributed to one of the research group’s main objectives, to develop the methane-free production of fertiliser raw materials (ammonia, ammonium nitrate or urea) using water, air, carbon dioxide and ultrasonic media.
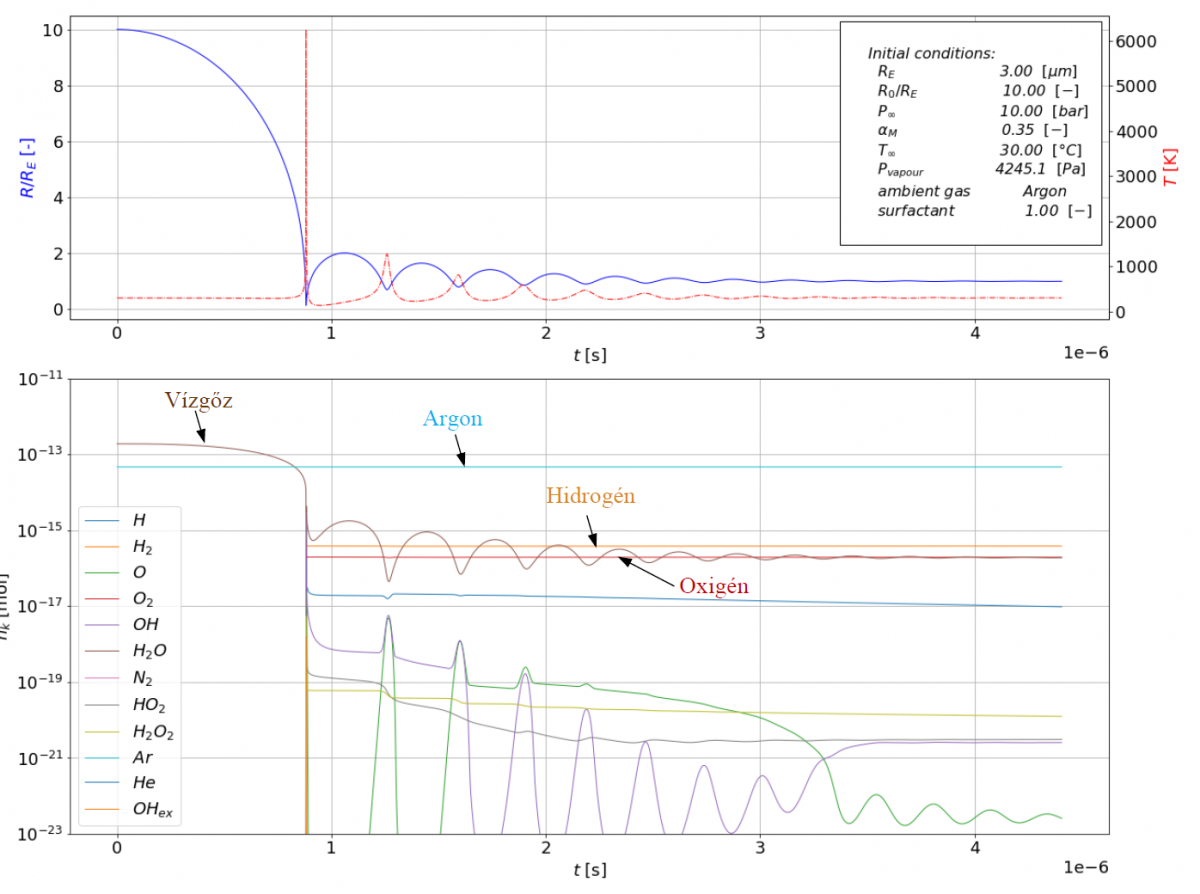
Illustration of a simulation: blue indicates the bubble radius, red the temperature with the amount of chemicals below. (Source: Kozák Áron TDK pályamunkája )
Energy costs pose one of the main engineering challenges in hydrogen production today. Currently, most hydrogen is produced from hydrocarbons, for example by the steam reforming of natural gas, which also produces significant carbon-dioxide emissions. The non-renewable resources used for the process are imported from outside the EU, so the researchers are interested in greening the hydrogen production process.
Áron Kozák said, that he defines the energy needed to vibrate the bubbles during his theoretical calculations, and uses numerical simulations to determine the amount of hydrogen produced in the bubbles. The division of these two values gives a result that can be directly compared with other methods and with the combustion heat of hydrogen. Áron used a model, which examines only one spherically symmetric bubble and can be represented by an ordinary differential equation. “Despite its apparent simplicity, it is a highly complex model. I have broken down the process into nearly 60 equations in my paper, because in addition to bubble dynamics, calculations are needed for thermodynamics and evaporation, and the heat conduction at the bubble wall must also be considered, not to mention the 29 reaction between 11 compounds in the reaction mechanism. The differential equations describing the processes have a so-called rigid behaviour, i.e. their numerical solution is computationally intensive. The task is further complicated by the fact that the simulation have to be run several times after refining and adjusting certain control parameters (e.g. bubble size, excitation rate, ambient pressure, temperature, etc), to optimise the calculations,” Áron Kozák explained the details of his theoretical research.
The young engineering student added that the process had not been applied in industry due to the energy efficiency issues to be addressed. The research team is now working on improving these issues. Kozák emphasized that hydrogen also plays an important part in a sustainable future, as the European Union has set a target of producing 10 million tonnes of hydrogen in a fully CO2-free production internally and of importing just as much by 2030. There will also be a need for peak power plants and energy storage facilities, for example, to compensate for the fluctuations in renewable energy output caused by changing weather patterns. In addition, hydrogen can also be used as a green fuel, especially in larger vehicles where lithium-ion batteries are less suitable (e.g. trucks, trains, planes). Kozák added, that experts are exploring the hypothesis that hydrogen could also make steel production, currently responsible for 7% of global emissions, carbon neutral. Hydrogen is also an indispensable ingredient in agriculture: it is one of the main ingredients in fertiliser as a component of ammonia.
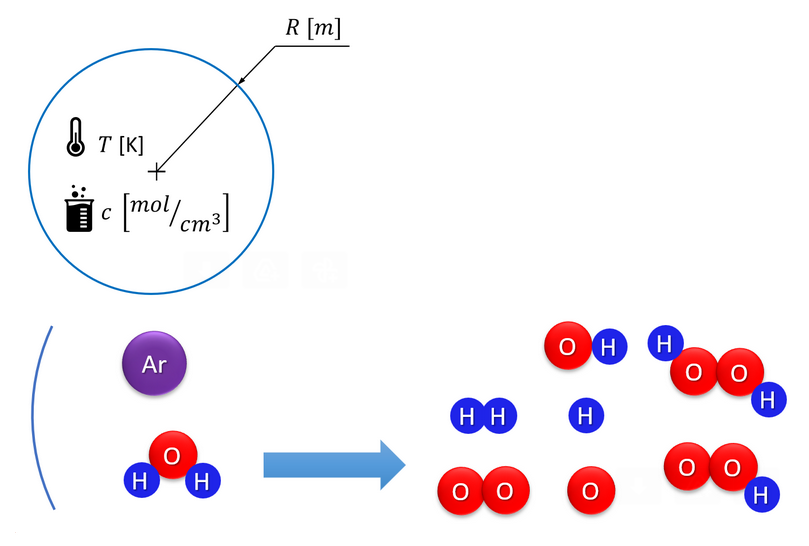
Part of a presentation by Áron Kozák, showing the processes involved in hydrogen production
Reflecting on the seriousness of the research topic, the award-winning student said that there was a lot of competition in the research of hydrogen production by water decomposition. Electrolysis is the most common process among professionals, which currently has an efficiency of 70% (slightly higher under laboratory controlled conditions).
Thermochemistry also plays a significant role in hydrogen production. The process, like the one inside bubbles, involves the decomposition of water into hydrogen and oxygen elements when exposed to temperature. In order to protect the equipment that carries out the reaction, some metal oxide is also involved: it is introduced into a closed circuit so that the process can take place at lower temperatures. The advantage of this method is that it produces hydrogen directly from heat (for example in a concentrated solar power plant or a nuclear power plant).
"It has not yet been used in the chemical industry, but there are existing industrial applications for ultrasonically excited bubbles called acoustic cavitation. They are used for example for waste water treatment and sterilisation in the food industry, and clinical trials have also started recently for an ultrasonic cancer treatment for cancer types that cannot be cured by chemo- and radiotherapy," said Kozák, adding more interesting details about the subject. He said that he was planning to write his bachelor thesis on the details of this research topic, and that he was also keen to contribute to scientific publications on the subject. Currently, he is working on converting the software code of his computer program into a format that will be accessible to others and useful for their own research.
Áron Kozák will soon complete his undergraduate studies at the University of Technology. He is intent on continuing his studies, but he has not yet decided whether he would do so at home and at the University of Technology in the Master's programme in mechanical modelling, or abroad. He now thinks he wants to find a job in the industry after graduation. “I am open to all sorts of possibilities, and we will see what the future holds. Right now I am focusing on my master programme, as I definitely want to continue my studies,” he concluded his interview to bme.hu.
TZS-KJ
Photo sources: NOVOFER Foundation and Áron Kozák

