News feed
Eye Treatments Could Become Simpler Thanks to Tibor Kapu’s Space Experiment
2025. 07. 02.
A unique device developed by lecturers at BME could replace the need for inconvenient, multiple daily applications of eye drops.
Anyone who has been following the story of Hungary’s second astronaut — whose launch was postponed several times for technical reasons — will know that within the HUNOR Programme, Tibor Kapu, a research astronaut and former BME student, together with the Ax-4 mission crew, is carrying out a range of experiments on the International Space Station. While most of these are physics-related, one has particular medical promise: the test of a Hungarian-developed device that could open up new possibilities in ophthalmic care.
What makes this especially interesting is that the invention by Spinsplit Ltd. — developed in partnership with BME and Semmelweis University — was originally designed to tackle a major problem in space travel, but if all goes well,
it could make certain treatments much simpler and more efficient back on Earth, too.
Without targeted intervention, most astronauts develop noticeable vision problems — and on longer missions, this can even lead to lasting eyesight deterioration. This is mainly due to the redistribution of body fluids in weightlessness: the amount of fluid in the upper body increases, causing the blood vessels to expand and intracranial pressure to rise, which in turn can lead to oedema in the eye, compressing the optic nerve. This condition is known as Spaceflight Associated Neuro-Ocular Syndrome (SANS), which NASA ranks among the five most critical health issues of spaceflight.

It does matter whether the astronaut sees clearly or blurry
While SANS could potentially be prevented in future with medication, delivering drugs precisely to the eye in zero gravity is anything but straightforward. Traditional liquid eye drops don’t work without gravity; only complicated flushing procedures can reach the affected area.
But those methods are impractical and unreliable — and they require extra equipment, which space crews try to avoid whenever possible.
By contrast, imagine a soft object just a few millimetres in size that can be removed from a finger-sized device in a single motion and placed in the lower conjunctival sac, where it dissolves and takes effect.
In its current research phase, this ophthalmic insert is a sterile, polymer-based, nanofibre device without an active ingredient. The revolutionary technology was developed by BME researchers at Spinsplit Ltd., led by Diána Balogh-Weiser (Department of Organic Chemistry and Technology) and Ferenc Ender (Department of Electronic Devices), in close collaboration with institutes at Semmelweis University (Department of Ophthalmology and Faculty of Pharmaceutical Sciences) under the professional guidance of Professors Zsolt Zoltán Nagy and György Tibor Balogh. The R&D work forms part of the END-SANS project within the HUNOR Hungarian Astronaut Programme.
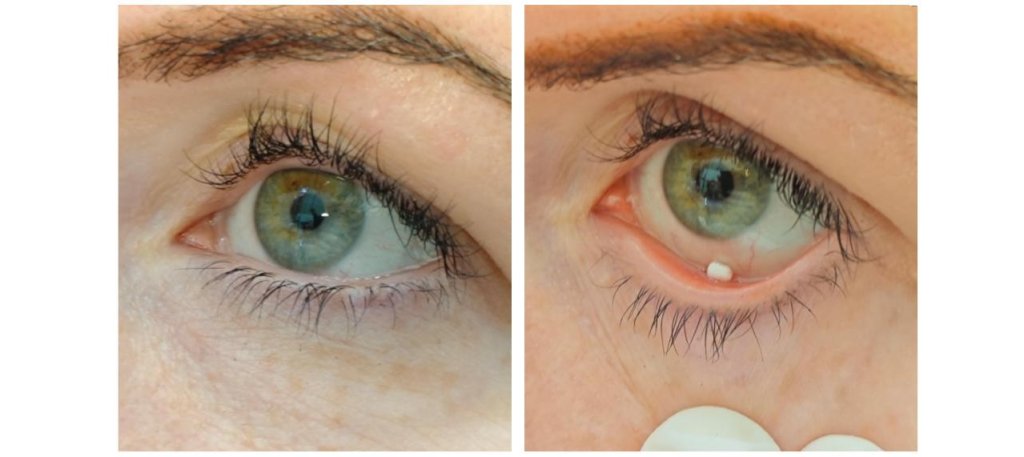
That's where it needs to be put in
The company, now BME’s official innovation partner, is a fortunate meeting point of the mindsets of the Faculty of Electrical Engineering and Informatics (VIK) and the Faculty of Chemical Technology and Biotechnology (VBK). As the two founders put it, it’s the engineering and technological realisation of an idea rooted in pharmaceutical science. It’s a prime example of how different scientific fields can work hand in hand. “We are working on projects that do not fit within the classic university framework, yet
we consciously aim to set good examples for the operation of a future university innovation ecosystem.”
“As a department, we specialise in ‘miniaturising systems’. A branch of this is microfluidics, where we shrink fluidic systems to the micro scale. In these tiny environments, the high surface-to-volume ratio lets us control conditions like temperature and flow rate very precisely, so experiments are more repeatable. Building on this expertise, our team at Spinsplit first began developing nanofibre technology in 2018 — basically creating a liquid–solid phase transition in a microenvironment,” Ferenc Ender told bme.hu.
He and Balogh-Weiser first crossed paths through BME’s biomedical engineering training programme. After both completed their PhDs, they realised it made sense to set up a company to make their system more widely accessible. They joined funded research projects and were soon joined by nanotechnology experts, allowing them not only to offer devices but also their expertise in nanofibre production as a service.

Ferenc Ender and Diána Balogh-Weiser
“It was around that time we learned there would be a new Hungarian astronaut — though nobody knew yet who — with medical support provided by Semmelweis University. So Zsolt Zoltán Nagy, Director of the Department of Ophthalmology, dove deep into the literature on astronauts’ eye conditions. We had already worked together; Semmelweis had purchased several of our fibre-forming devices. So together we submitted a grant proposal focused on ophthalmology in space,” Balogh-Weiser told bme.hu.
NASA Is on Board Too
In less than a year from the initial idea, they have reached the point where an astronaut is now testing their nanofibre-based ophthalmic inserts during a mission capturing national attention for two weeks. “We’ve had the full backing of the HUNOR Programme, both professionally and operationally. NASA and Axiom Space have also responded positively to the project, which could offer a practical solution to an everyday problem encountered during space travel,” she added.
The research astronaut has received sterile, pen-like applicators containing the inserts, which can be placed in the eye like a contact lens. The insert has a texture similar to cotton wool; when it comes into contact with the moisture of the eye surface, it dissolves in a controlled manner and may cause a foreign-body sensation only briefly. The Ax-4 mission test focuses on usability: whether the device remains sterile, can be opened with ease, can be applied by the astronaut, and whether it causes discomfort or impairs vision. The medical device must meet strict protocols set by both Hungarian authorities and NASA.

Az applicator
Semmelweis University experts examined the research astronaut’s eyes before the mission and will do so again afterwards. Meanwhile, a control group of volunteers on Earth is testing the technology at the same time, ensuring a controlled study — a must for any new clinical product or device.
If the tests prove successful, the inserts have great potential for everyday use too: instead of applying eye drops five or six times a day, a single insert could suffice. It would also allow active ingredients to be stored for longer in a stable form. “You can dose the active substance much more precisely than with drops — people often miss the eye or blink it out, and tears dilute the medication about fifteenfold.”
No More Constant Eye Drops
The biggest drawback of eye drops is the unpredictable absorption of the active ingredient, and their application is often inconvenient. In contrast, with a solid insert, the release time can be precisely controlled: it can be designed to dissolve immediately or over several hours. “This means you can keep the active substance on the eye’s surface for hours without needing to apply more every few hours. That’s a great relief not just for patients treating themselves at home, but also for those being treated in hospital,” Balogh-Weiser explained.
Another challenge is that many drugs don’t dissolve well in water and can’t be stored for long, even when frozen. Solid formulations contain minimal water, so the drug stays in a more stable state, making longer storage and better stability possible.
Eye drops are unlikely to disappear altogether, but solid carrier systems will probably play a big role in the future of ophthalmic treatments.
The current plan is to develop the invention, in collaboration with BME, up to the point where it can enter large-scale pharmaceutical development, ideally with the help of a major manufacturer. “We plan to move forward in collaboration with InnoLab, BME’s technology and knowledge transfer company. Becoming BME’s innovation partner was the first step in this process — our goal is to carry out the next stages of technology development in close partnership with the university,” said Ferenc Ender.
When asked about the patenting status of the END-SANS Team’s innovation, he noted that space patenting is a highly specialised area requiring ongoing coordination with patent attorneys and the partner universities involved.
pg
